TMS Therapy in Lake Mary, FL
TMS (Transcranial magnetic stimulation)
Primary Care / Family Medicine, Counseling and
Psychiatry brought together – Whole Person
Healthcare
TMS Therapy
Typical Conditions and Treatments
Although many different forms of drug therapies exist, these may be unsatisfactory due to a lack of effectiveness or undesirable side effects. Transcranial Magnetic Stimulation, or TMS in short, can help achieve remission in major depressive disorder as an alternative, FDA approved treatment for patients that did not benefit from prior antidepressant medications.
FDA approved alternative to drug therapy
Although many different forms of drug therapies exist, these may be unsatisfactory due to a lack of effectiveness or undesirable side effects. Transcranial Magnetic Stimulation, or TMS in short, can help achieve remission in major depressive disorder as an alternative, FDA approved treatment for patients that did not benefit from prior antidepressant medications.
TMS may help achieve remission in major depressive disorder
- Non-invasive, meaning it does not involve surgery, anesthesia or sedation of any kind.
- Non-systemic, meaning it is not a pill that has to be swallowed and does not circulate the blood stream.
CAYA HEALTH
What to expect
Each TMS treatment session takes approximately 20 to 40 minutes. Before and right after treatment, the patient can continue with their normal everyday activities. In total, the patient receives 5 therapy sessions for 4 to 6 weeks. Patients are alert and awake during treatment.
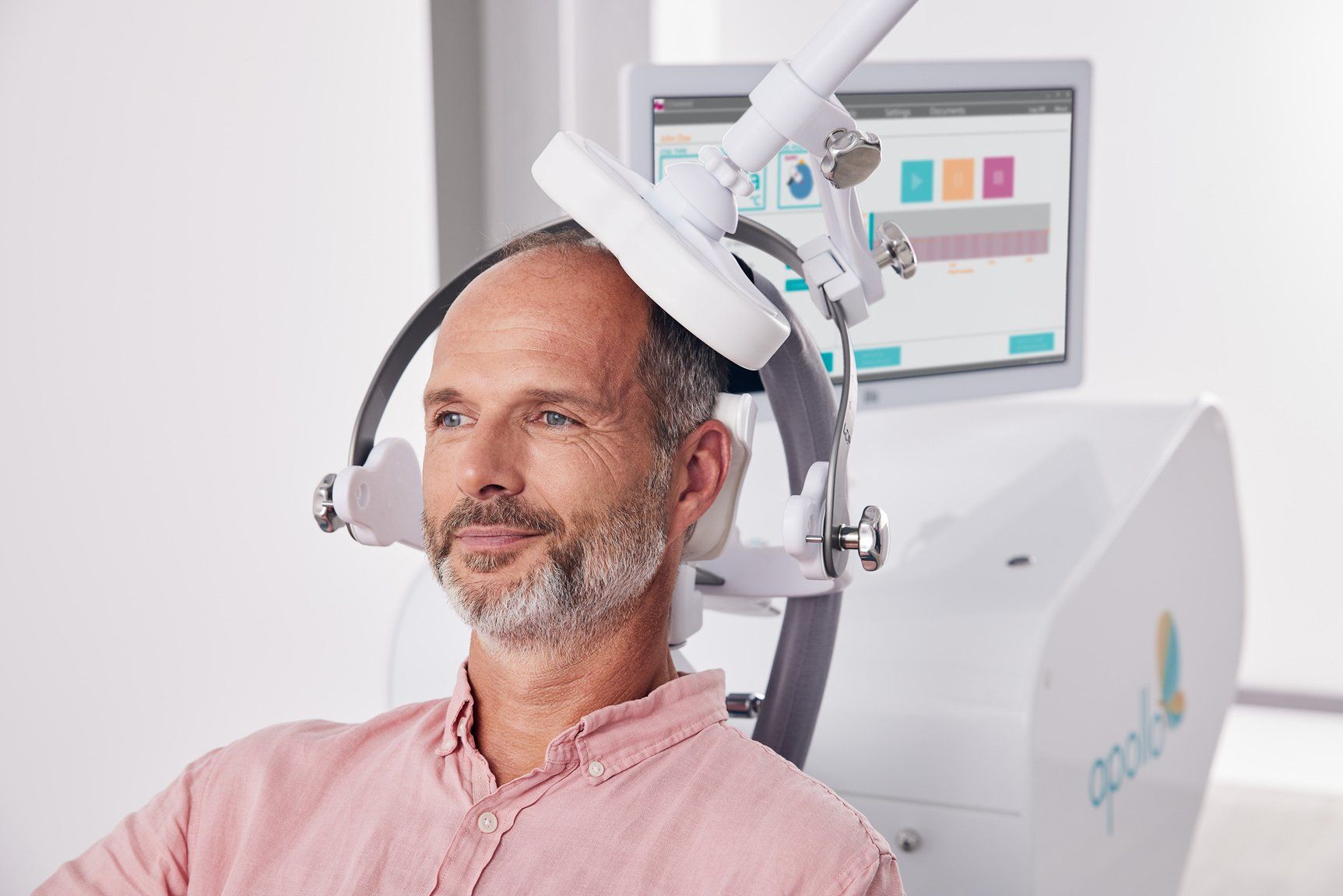
Controlled clinical trials applying TMS on patients who had failed to achieve satisfactory improvement from one prior antidepressant medications showed that:
- Approximately 1 out of 2 patients experienced significant improvement in depression symptoms.
- Approximately 1 out of 3 patients experienced complete symptom relief after the full TMS therapy.
More Information
Please ask your doctor if TMS is right for you. You should not apply TMS if any of the following conditions apply:
- You have implants such as cochlear implants, internal pulse generators, medication pumps, pacemakers or other implanted devices.
- You have metal of any kind in the head or brain area.
- You have a history of epilepsy.
- You have had a vascular, traumatic, tumoral, infectious, or metabolic lesion of the brain.
- You are pregnant.
TMS is a safe form of treatment that has been cleared by FDA since 2008 for patients with depression who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode. If you are unsatisfied with your current depression medication or worry about its side effects and are looking for a proven, drug-free alternative, ask your doctor about TMS.
Caya Health - Whole Person Integrated Healthcare
Lake Mary Office Location
1355 S International Pkwy, Unit 1481, Lake Mary, FL. 32746
Appointment Phone Number
Fax Number
If You Have A Question Or Need An Appointment
We accept
All Rights Reserved. Terms and Privacy Policy | Bizimage Marketing







